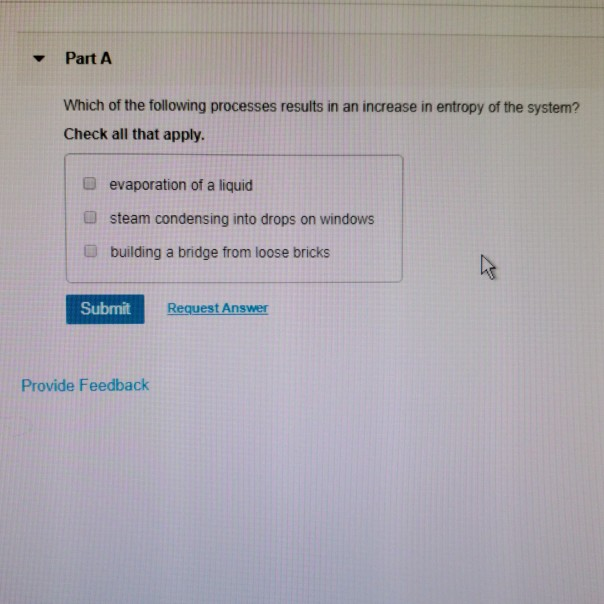
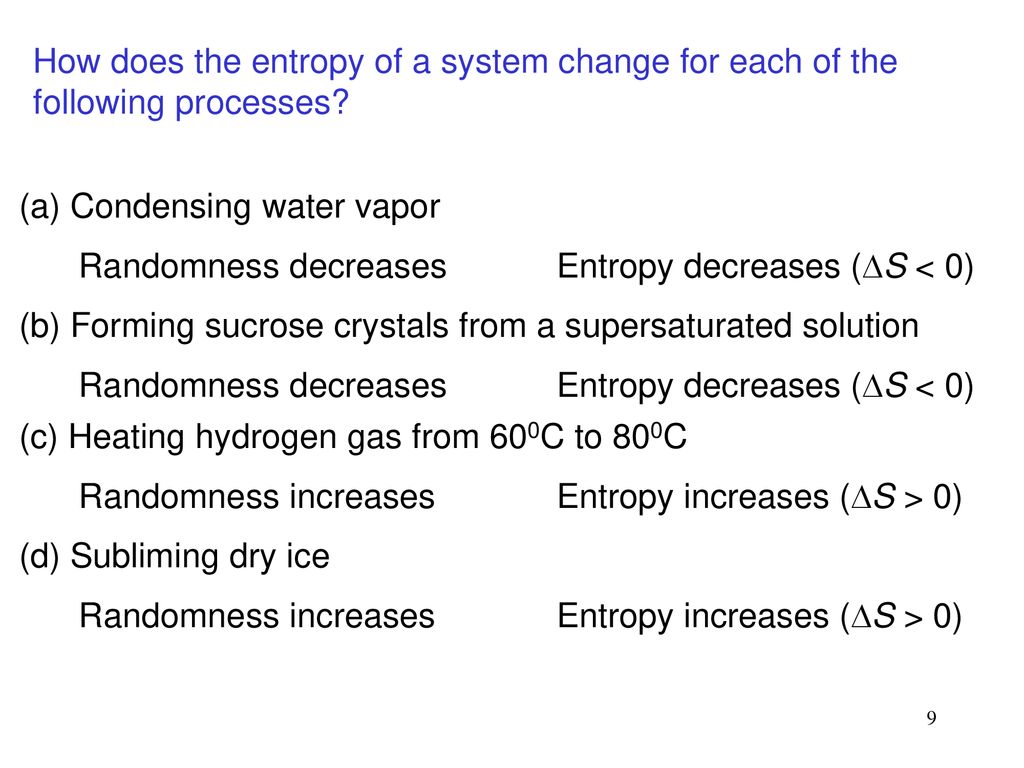
Which Of The Following Processes Results In An Increase In Entropy Of The System?
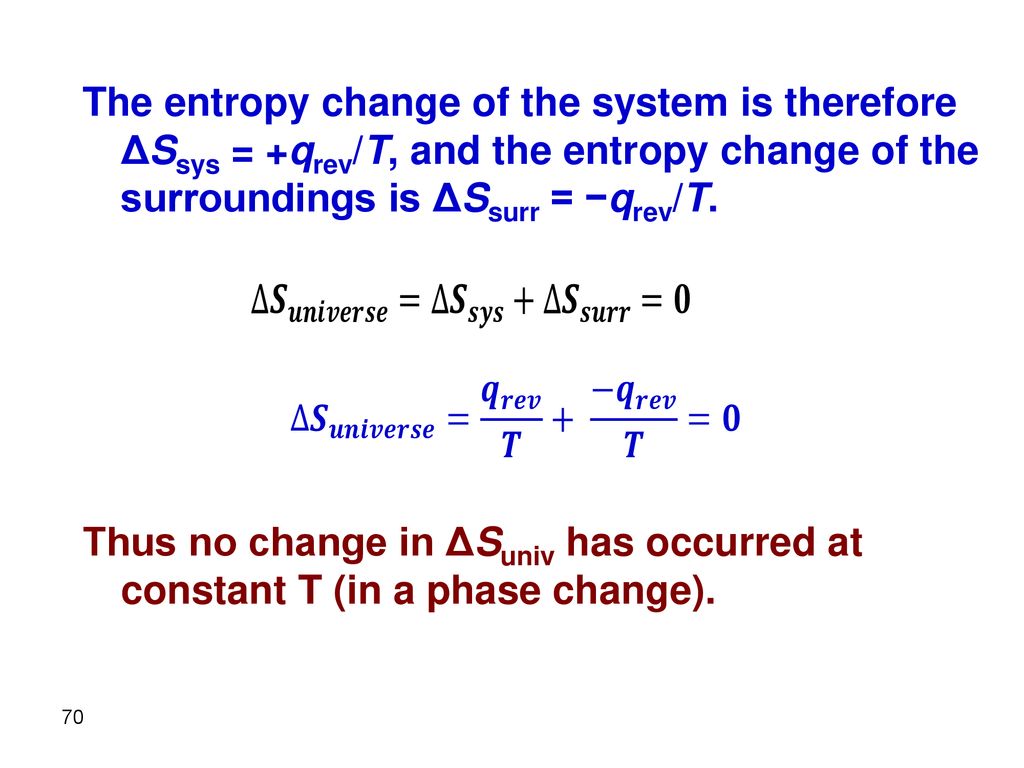
Which of the following processes results in an increase in entropy of the system?. In an isolated system chemical reactions occur in the direction that leads to an increase in the disorder of the system. It is only a closed system if we include both the gas and the reservoir. So here we are asked to determine the change in a certain state wanted a state function quantity.
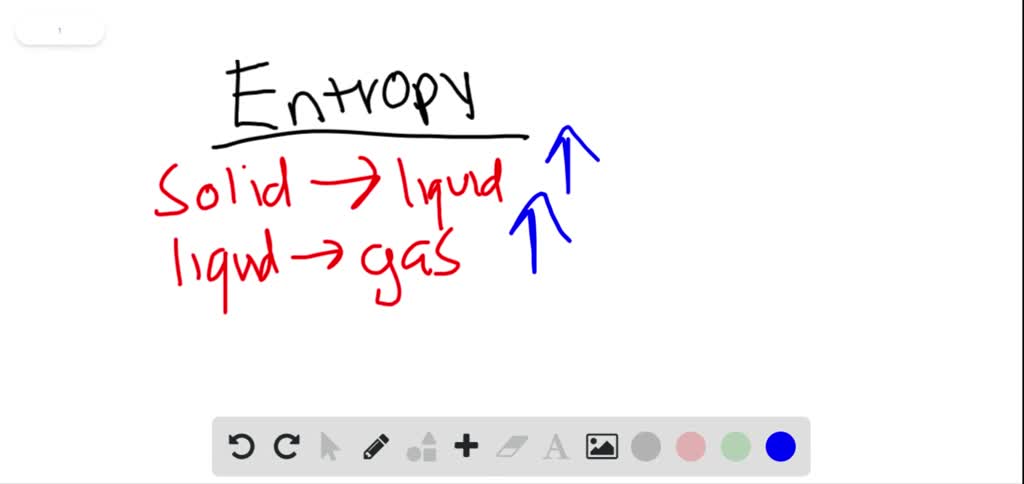
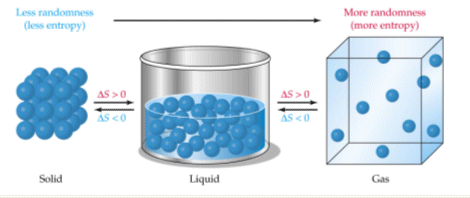
When solids dissolve in liquids they also gain freedom of motion and experience an increase in entropy. Thus we can conclude that melting of a solid evaporation of a liquid sublimation mixing and diffusion involve an increase in the entropy of the system under consideration. Now we know that liquid has more entropy then solids due to their more free form.
Each degree of motion increases the number of available microstates resulting in a higher entropy. So randomness is increasing. On other hand if Δn n products - n reactants o it willdecrease entropy.
B Any irreversible process results in an overall increase in entropy. Diffusion is a process in which molecules are able to rapidly move from one place to another. General TypesGroups of problems.
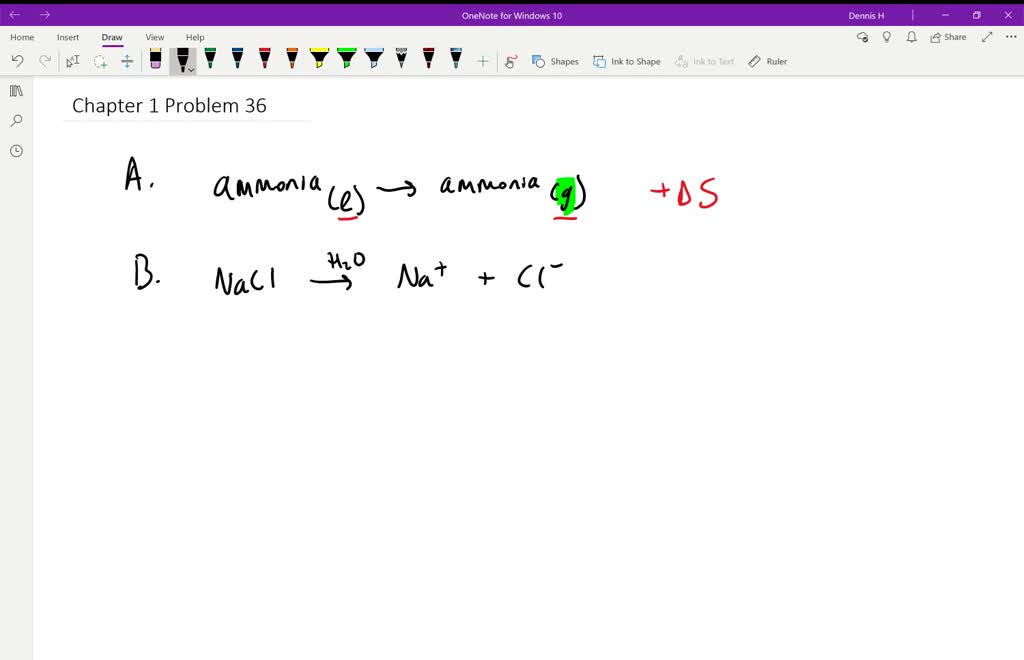
Chemical reactions also tend to proceed in processes that tend to increase. Second Law of Thermodynamics. The dissolution of sodium chloride in water.
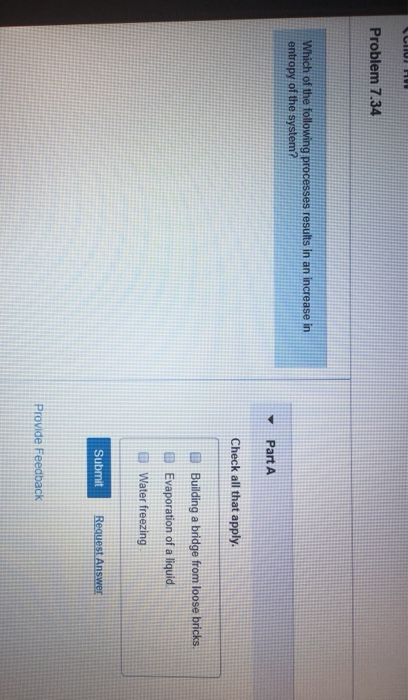
Evaporation of a liquid. D Entropy increases with the number of microstates of the system. The evaporation of ethanol.
Similarly when a liquid is converted to a vapor the greater freedom of motion. The more randomness in a system means the entropy of the system is higher.
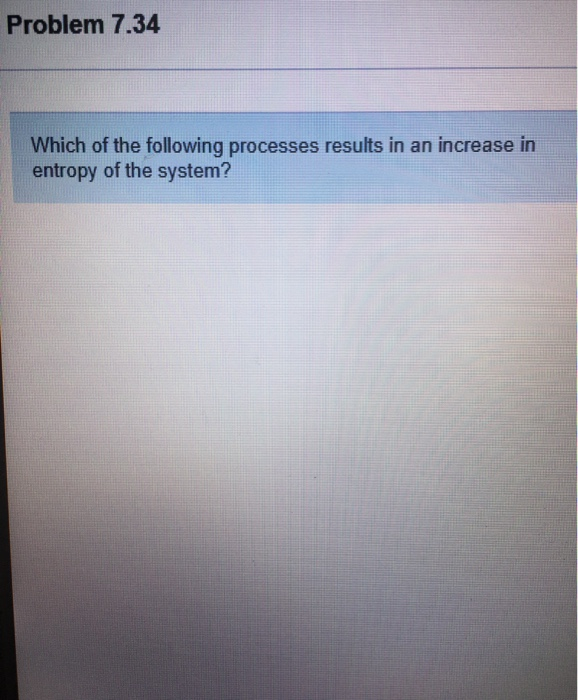
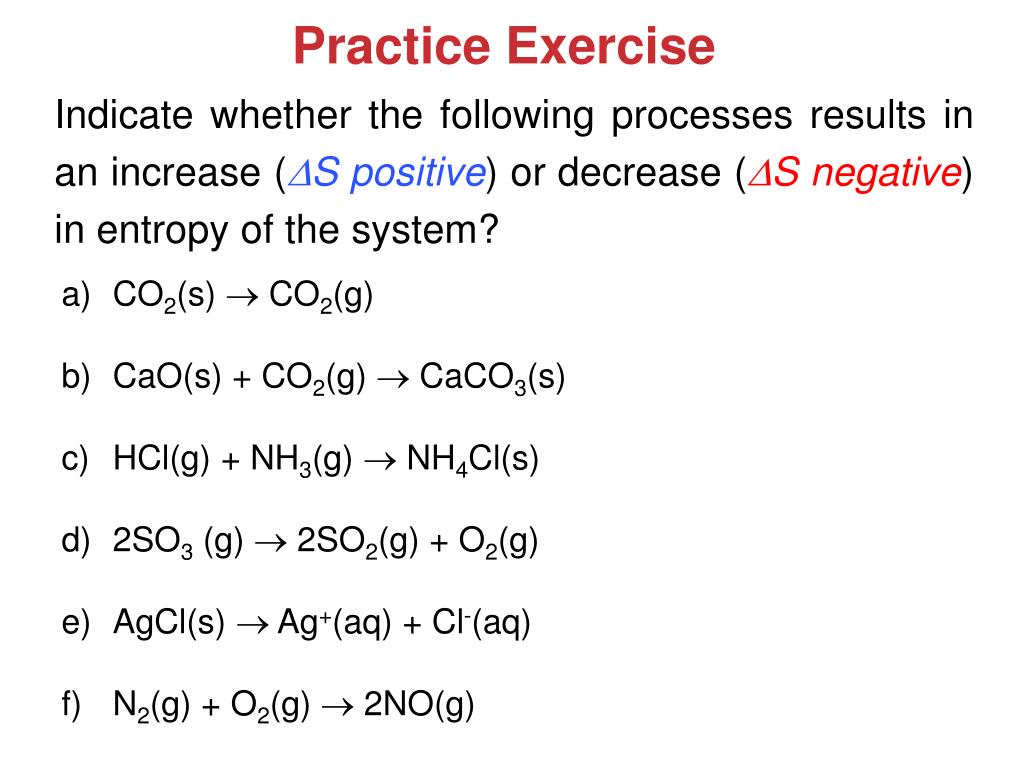
Problem 734 Which of the following processes results in an increase in entropy of the system.
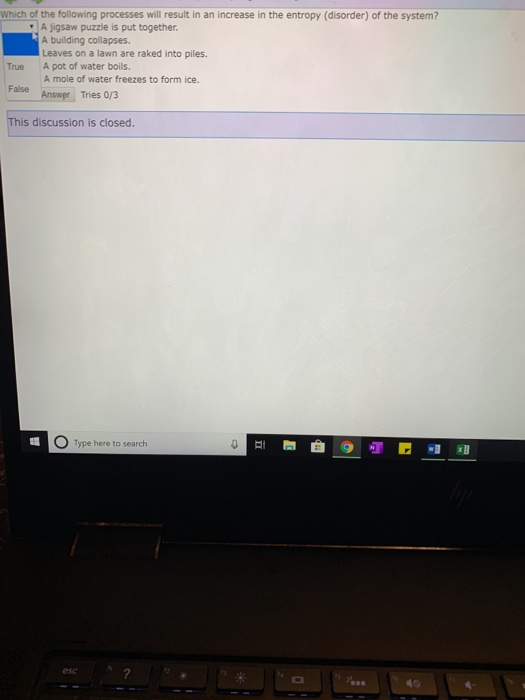
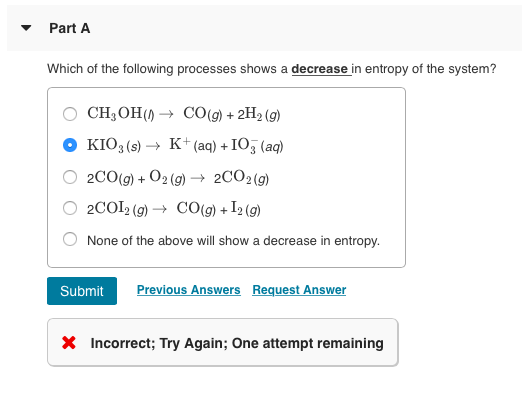
A Preparing aqueous solution of common salt. When the physical change results in a more disordered state then entropy is increased. The total entropy of the universe increases in any spontaneous process. The melting of candle wax. 1 Which Of The Following Processes Will Result In An Increase Of Entropy Of The System. So randomness is increasing. Extra Practice Problems. The surroundings increase entropy. The dissolution of sodium chloride in water.
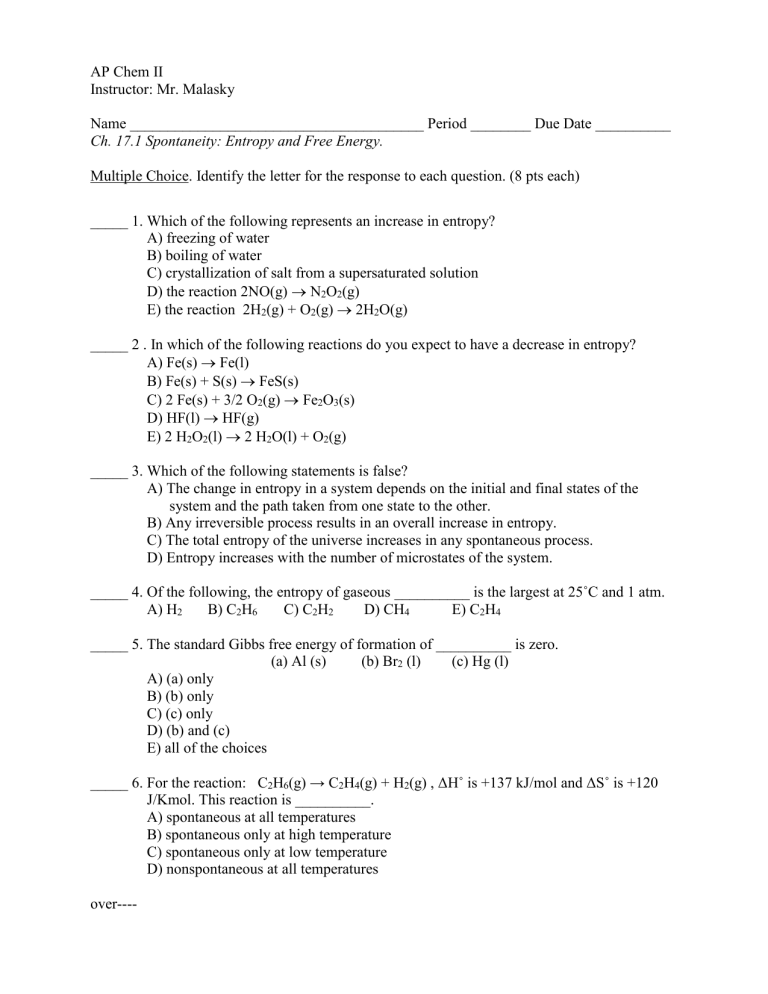
The surroundings increase entropy. Evaluating Relative Molar Entropy for Chemicals Calculatingp1 ΔGfor Reactions Math p5 Evaluating ΔS for Reactions non-math p2 ΔG ΔH ΔS Equilibrium and Temperature p6 Calculating p2ΔS for Reactions Math Answers p7. The total entropy of the universe increases in any spontaneous process. On other hand if Δn n products - n reactants o it willdecrease entropy. So first were going to consider the melting of the Ice Cube. When the physical change results in a less disordered state then entropy is decreased. The melting of candle wax.












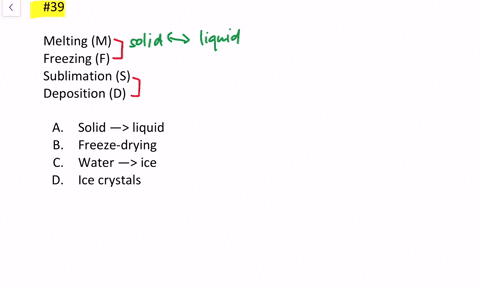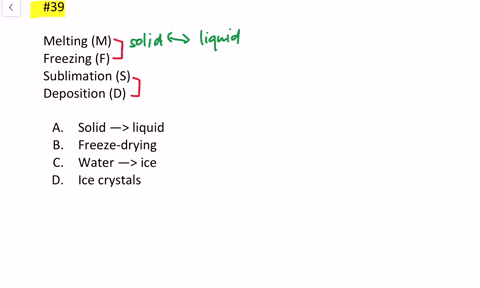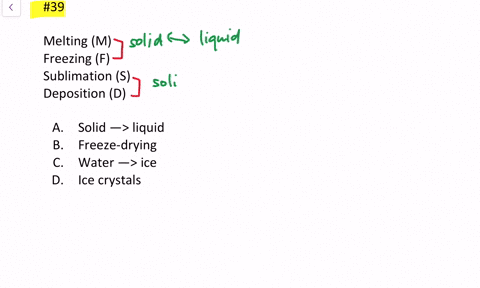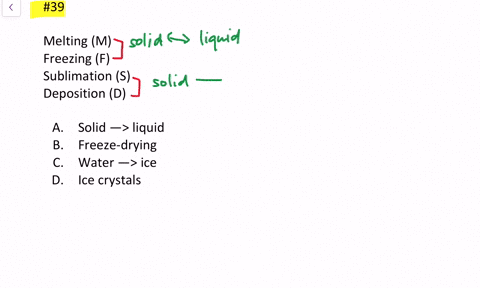

















Post a Comment for "Which Of The Following Processes Results In An Increase In Entropy Of The System?"